How a Tiny Insect Was Used to Trace the Origins of Human Clothing
Important clue: The head louse is a bloodsucking insect that lives only on the scalp – but the body louse doesn’t venture towards the head.
Humans are the only species that wears clothes. And it is obvious that we are also obsessed with our clothes, with their designing, making and procuring. However, how did it all come about? For example, when did we start wearing clothes?
The trouble with this kind of investigation is the lack of hard evidence. Clothes, unlike bones, do not fossilise and, unlike stone and metal, perish quickly. So except under special environmental conditions in which some palaeo-humans (such as the iceman Ötzi) have been unearthed, direct evidence of prehistoric clothing has remained scant and the origins of clothes have been lost in the mists of time.
But scientists were able to find a workaround in the form of a small insect.
The pest family
Almost all mammalian and avian species are host to various species of lice. But humans are among the few species that are host to three (or subspecies). The human head louse (Pediculus humanus capitis), the body louse (P. humanus corporis, also considered P. humanus humanus) and the pubic louse (Pthirus pubis) are obligate ectoparasites to the human body and can't survive on other species, including pets.
Head louse are slightly smaller in size than body louse and usually have a darker pigmentation. There are subtle differences in the lengths and widths of their antennae and front legs. But not surprisingly, the head louse and body louse have considerable morphological similarities. The principle difference lies in their choice of habitat.

Lice that paracitise humans: (L-R) head louse, body louse and pubic louse. Credit: Wikimedia Commons
The head louse is a bloodsucking insect that lives only on the scalp and lays eggs only on scalp hair. The body louse, on the other hand, doesn’t venture towards the head. It feeds off of the human skin. But perhaps more notably, it lives and lays eggs in human clothing.
The head louse and the body louse are fastidious about their habitats: neither encroaches into the other’s territory (in fact, the head louse can't live on clothes). Neither species can survive away from a human host for long: the head louse perishes within 24 hours, while the body louse can live without human contact for only a week. Mark Stoneking, now a scientist at the Max Planck Institute for Evolutionary Anthropology, Leipzig, took interest in the migration of these obligate parasites across the globe, hoping they would lead him to clues about human migrations themselves.
He hypothesised that the head louse was an ancestral species and that the body louse evolved from the head louse when a new ecological niche got available: the folds and creases of human clothes. When could this have happened? The likeliest answer is when humans started wearing clothes regularly.
This leads to the intriguing possibility that zeroing in on the time when the body louse evolved from the head louse would by inference correspond to the beginning of extensive use of clothing by ancestral human populations. To get at this, Stoneking and his peers use a molecular clock.

Mark Stoneking, a researcher at the Max Planck Institute for Evolutionary Anthropology. Credit: Eugene Dubois Foundation, The Netherlands
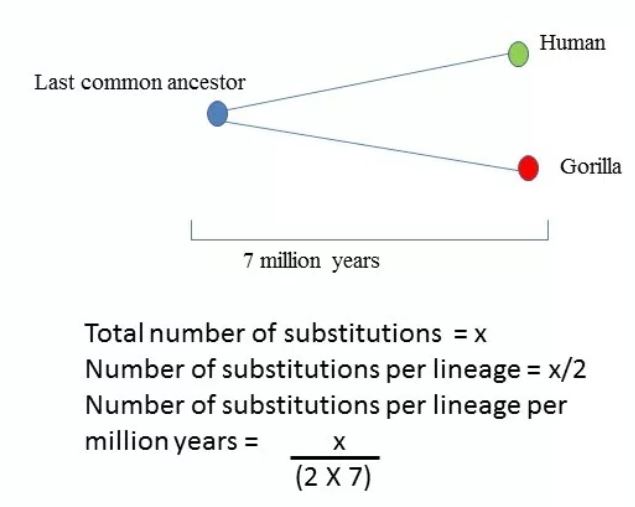
The molecular clock is a method to determine when two biological species diverged from a common ancestor. It is based on the principle that, as time passes, random errors and/or substitutions creep into the species's DNA when it replicates during reproduction; these 'errors' get transmitted down generations. So the longer it has been since two sequences diverged, the more the number of differences between their DNAs.
This problem is further simplified if the value of a single parameter can be computed: the number of substitutions per million years. This can be calculated using the number of substitutions that have accumulated in two DNA sequences whose divergence-time is actually well-known from other lines of evidence (e.g., from the fossil record). Assuming the error rate stays constant across time and in different species, this allows scientists to calculate the unknown time points.
Truth and lice
The molecular clock for dating lice evolution was built using two mitochondrial DNA and two nuclear DNA segments. To avoid any bias in their investigation, the scientists collected lice from 12 geographical regions – Ethiopia, Panama, Germany, Philippines, Iran, Ecuador, Laos, Papua New Guinea, the US, Taiwan, Nepal and the UK – and extracted their nuclear and mitochondrial DNA. They also collected DNA from chimpanzee head louse.
Since it is well known that hosts and their parasites often coevolve, it was assumed that the chimpanzee louse (Pediculus schaeffi) and P. humanus must have co-speciated with their respective hosts and that this must have happened around 5.5 million years ago – the time when humans and chimps also diverged. Thus, the differences between DNA sequences of chimp louse and human head louse must have accumulated over 5.5 million years. Using this duration, the time when the head louse and the body louse diverged was estimated.
Stoneking and his team analysed DNA from both the nucleus as well as the mitochondria of lice. First, they analysed the DNA sequence of two genes – ND4 and CYTB – present only in mitochondrial DNA. Next, they studied the sequence of two important nuclear genes – EF-1α and RPII. It was not necessary to sequence them completely; a few fragments of 400-600 base pairs each sufficed.
The first result clearly showed that the African louse (collected from Ethiopia) was genetically more diverse than the non-African lice. The finding mirrored the greater genetic diversity of humans seen in Africa compared to those in other continents. Since greater genetic diversity almost invariably occurs at the source, the results indicate the African origin of the human louse.
Similarly, they found that the human head louse was more genetically diverse than its cousin, the body louse – proving that the head louse was the ancestral species.
Next, a phylogenetic tree was constructed using all the mitochondrial sequences of human lice. This is a tree-like diagrammatic representation that describes the evolutionary relationships between the organisms being studied. It showed the presence of a number of clades; a clade is a group of all sequences used in a study that have descended from the same ancestral sequence. The deepest clades contained only head louse sequences, confirming that the body louse had originated from the head louse.
One clade in particular contained all the body lice and 16 head lice sequences, and included samples from all over the world. The molecular clock showed that this clade was 32,000-112,000 years old. Since it contained all the body lice sequences, the estimated age of this clade had to form the upper limit for the time since the body louse first evolved. And since the body louse exclusively inhabits human clothing, this must have been the time when modern humans started wearing clothes regularly.
New ecological niche

Phylogenetic tree based on mitochondrial DNA sequences, showing divergence of head louse (H) and body louse (B). The arrows indicate the estimated ages of the clades. Note that one clade, who age is ~72000 years, houses all body louse sequences and some head louse sequences. Adapted and simplified from Kittler et al (2003) Curr. Biol. 13, 1414-1417
As a final piece of evidence, Stoneking’s team also analysed the sequence of a different gene present in mitochondrial DNA and showed that the results agreed with those obtained from the ND4-CYTB analysis. In other words, it was starting to look as if anatomically modern humans started wearing clothes around 70,000 years ago.
In a subsequent test, David Reed and his colleagues from the University of Florida, Gainesville, performed a more robust analysis and concluded that the body louse had diverged around 170,000 years ago and certainly not after 83,000 years ago. The difference between the two sets of data is not surprising given the different methodologies used. However, they are in broad agreement. Both conclude that the body louse evolved in the presence of anatomically modern humans in Africa because of the availability of a new ecological niche: clothes.
What if clothing originated much earlier and if the louse had colonised this ecological niche later? This possibility can't be entirely discounted. Stoneking believes that, since a new ecological niche is colonised fairly rapidly, it is unlikely that clothing could have existed for thousands of years before the body louse marched upon it. The genetic data also corresponds well with the archaeological finding that the earliest eyed needles – the only prehistoric tools that can be definitely associated with clothing – are about 40,000 years old, and they have been found only in settlements of modern humans, not among archaic humans like the Neanderthals.
In sum, the genetic and archaeological data both converge on the conclusion that the chimp louse and the human head louse are close cousins that must have originated from a common ancestor. The human head louse was confined to one relatively smaller habitat (the scalp), when ancestral humans lost a significant amount of body hair about 1.2 million years ago. Then, sometime between 70,000 and 170,000 years ago, anatomically modern humans started stitching and wearing clothes and the lice could now colonise them. Indeed, it is quite possible that human lice spread across the globe along with their humans.
Anirban Mitra is a molecular biologist and a teacher residing in Kolkata. He would like to thank Mark Stoneking, the Eugene Dubois Foundation and Club SciWri for their contributions.
This article was originally published on Club Sci Wri. Read the original article.
This article went live on February eighteenth, two thousand eighteen, at twenty-nine minutes past six in the evening.The Wire is now on WhatsApp. Follow our channel for sharp analysis and opinions on the latest developments.