When Ribosomes Go Rogue
In the 1940s, scientists at the recently established National Cancer Institute were trying to breed mice that could inform our understanding of cancer, either because they predictably developed certain cancers or were surprisingly resistant.
The team spotted a peculiar litter in which some baby mice had short, kinked tails and misplaced ribs growing out of their neck bones. The strain of mice, nicknamed “tail short,” has been faithfully bred ever since, in the hope that one day, research might reveal what was the matter with them.
After more than 60 years, researchers finally got their answer, when Maria Barna, a developmental biologist then at the University of California San Francisco, found that the mice had a genetic mutation that caused a protein to disappear from their ribosomes — the places in cells where proteins are made.
This came as a complete surprise, says Barna: Everyone had expected the cause to be a mutation in a gene that orchestrated development, not one involved in ribosome structure. Ribosomes, which under the microscope look like millions of specks scattered across the cell, or — closer up — like a bread roll torn into unequal halves, appear similar in all life forms. They exist in every cell and were thought to do the same thing everywhere: translate instructions contained in DNA into the proteins that do most of the work in cells.
How could a defect in this dependable, ubiquitous little workhorse reshuffle the body plan of a mouse in such a curiously specific way?
Today, based on findings like these, a growing number of scientists have come to believe there’s more going on with ribosomes than they once thought — and that ribosome variety may sometimes have biological functions.
Barna, now at Stanford University, reported her finding in the journal Cell in 2011. Since then, researchers have uncovered several other genes that, like the one in “tail short” mutants, code for proteins in ribosomes and appear to distort development in a specific way when mutated.
In the unfortunate mouse that first intrigued Barna, a protein known as RPL38 was not being made correctly. In another mouse, a faulty version of a protein called RPL10A led to even more drastic (and deadly) deformities. “These embryos looked as if a guillotine had cut off their posterior end right after the hindlimb,” Barna recalls.
Picky ribosomes
There are other reasons researchers were surprised that unusual ribosomes lay behind the unusual development of these mutant mice. First, quality control on ribosomes is tight: Since faulty ones may churn out ill-conceived proteins that can do a lot of damage, they tend to be quickly eliminated. Second, embryos that have mutations in genes coding for ribosomal proteins generally don’t make it all the way through a pregnancy.
Yet there are exceptions, including in people. Children with isolated congenital asplenia, for example, are born without spleens, often due to a mutation in a single ribosomal protein, while the rest of the body is completely normal. Again — how could one missing or unusual protein in the ribosomes cause such a thing?
Barna believes that affinity is key. To make a protein, ribosomes don’t receive instructions directly from the unwieldy DNA, but from more succinct messenger RNA (mRNA) molecules that carry instructions about single genes. RNA is made up of a long string of four different building blocks, and every three blocks code for an amino acid. The ribosome reads these instructions and correctly joins the amino acids together to form a protein.
As far as scientists know, mRNA molecules just float around until they encounter a ribosome, at which point they get translated into protein. But Barna believes — and has evidence to suggest — that different ribosomes have different affinities and may be more apt to translate some mRNA types than others.
For example, the cells of mouse embryos with an Rpl38 mutation produce just as much protein as other embryos do. But they make substantially less of some proteins that are crucial during development. Known as homeobox proteins, they are essential for the embryo to sort out its back end. Barna discovered that ribosomes without RPL38 are less likely to bind to and translate homeobox mRNA. This results in a deficiency of these organizing proteins and perturbed development of vertebrae, ribs and tails.
Similarly, ribosomes without the ribosomal protein RPL10A are less likely to bind to mRNA that codes for another crucial set of embryonic development proteins — ones involved in what’s called the Wnt signaling pathway. Reduced numbers of these crucial proteins cause development to abruptly end after the hindlimb, creating the impression that the back end was cut off.
While some genetic disorders involving ribosomal proteins seem rooted in activities of unusual ribosomes, others may be due to ribosome shortages that result from the cell’s strict quality control, Barna says. Treacher Collins syndrome, which causes abnormalities of the face, and Shwachman-Diamond syndrome, which causes abnormal development of the skeleton, are likely examples.
Those are defects. But sometimes, Barna argues, differences in ribosome structure and composition may be functional. “We are discovering that certain ribosomal proteins occur more often in some cell types than others, say in neurons versus gut cells,” she says. Recent results from her lab suggest that there may even be different types of ribosomes within the same cell that specialize in making certain proteins over others.
In ribosomes, she says, “there appears to be a core that is invariable, and then in the outer shell, proteins that can vary within and between cells and tissues.” She believes this provides our body with yet another way of regulating which proteins get made, and where.
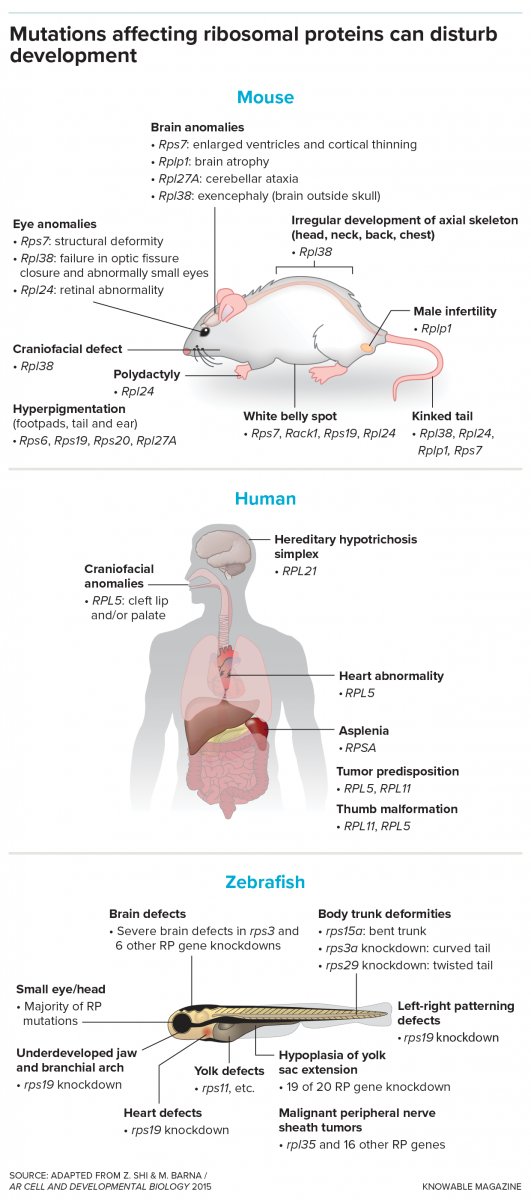
When ribosomal proteins are abnormal or missing, anomalies may arise.
Salty yeast
In November 2023, a two-day conference in London that Barna co-organized brought together many scientists who are intrigued by the variation they have found in the ribosomes they’ve looked at, and what, if anything, its functions may be.
Some aren’t quite so convinced that unusual ribosomes do anything beyond causing trouble. Katrin Karbstein, a biochemist at Vanderbilt University who coauthored a 2024 article about ribosome quality control in the Annual Review of Cell and Developmental Biology, notes that most of the known examples of ribosome variation cause disorders or disease. “Few if any have been demonstrated to be helpful to the organism,” she says.
Karbstein thinks that most genetic disorders associated with ribosome genes are probably just caused by the ribosome shortages that result when faulty ones are eliminated, rather than any special property of the divergent ribosomes themselves. If humans or mice end up with specific defects, she says, that may just be because low ribosome levels are a bigger problem in some cell types than others.
Yet in her own talk at the meeting, Karbstein revisited a discovery she herself made in the yeast cells she studies that, much to her surprise, did reveal a useful ribosome variant. When growing in very high salt concentrations, yeast cells lose a ribosomal protein, Rps26, from about half of their ribosomes. Ribosomes without the protein are different, Karbstein found. They seem more inclined to translate mRNA molecules that are produced in reaction to that stressor.
And when Karbstein and her team went on to deliberately remove Rps26 proteins from yeast cells, they found the cells became resistant to high salt. “In fact,” she says, “they now grow better under high salt.”
Karbstein also found that yeast responds nimbly to salt stress, quickly removing Rps26 proteins from ribosomes when needed and popping them right back in when the stressful situation subsides.
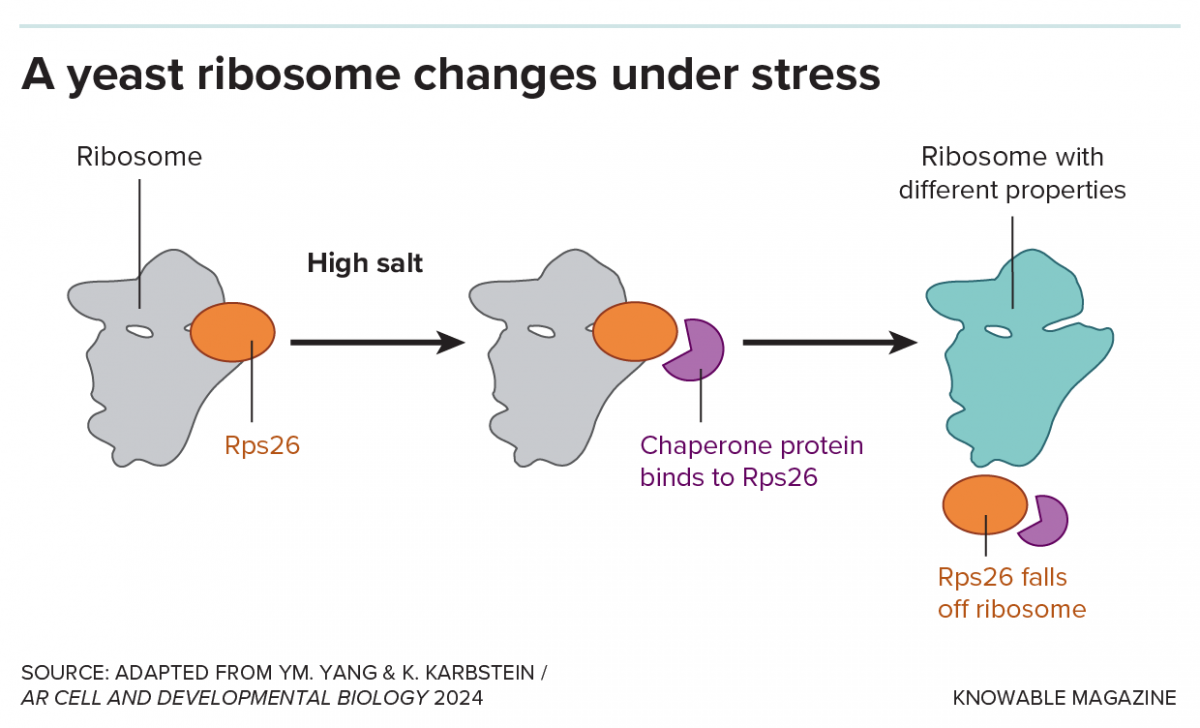
When growing in very high salt concentrations, yeast cells lose a ribosomal protein, Rps26, from about half of their ribosomes, with the assistance of what’s known as a chaperone protein. Yeast cells without Rps26 are better adapted to high salt.
Resistant ribosomes
Ribosomes aren’t made just from proteins: About half their structure consists of RNA. And in eukaryotes (life forms with complex cells like ours) there is plenty of extra RNA sticking out of the ribosomes, like the tentacles of a sea anemone. “We think this may provide a mechanism for trapping messenger RNA,” says Barna.
Ribosomal RNA also performs the most crucial and ancient function inside ribosomes: the fascinatingly efficient translation of mRNA into protein. The very first proteins must have been made by ribosomal RNA, argues structural biologist Ada Yonath of the Weizmann Institute of Science in Israel, who shared a Nobel Prize in 2009 for her work figuring out the structure of the ribosome.
Yonath notes that the pocket in ribosomal RNA where amino acids are joined together to form proteins looks very similar in all species, and she does not consider this to be a coincidence. “We think this is the proto-ribosome from which full ribosomes have evolved,” she says.
Ribosome researchers have long focused most of their attention on this neat, central area where mRNA is read and amino acids are joined together; they’ve spent less time studying the ribosomes’ outskirts. In addition, the ways in which ribosome structure has been studied created a strong impression that all ribosomes were the same. But new methods have uncovered more variation.
Yonath says she wants to see more evidence that differences between ribosomes can be helpful. Her lab is now collaborating with Barna and others to find out whether ribosomes that lack certain proteins or contain unusual ones have different three-dimensional structures that might explain why they work differently.
Yonath has long been interested in the differences between ribosomes of different species. These, she says, might be useful in developing antibiotics that target only the ribosomes of pathogens while avoiding significant damage to the beneficial microbes that live in our body, or to our own cells. “Over 40 percent of the clinically useful antibiotics target protein synthesis, mostly by paralyzing the ribosome,” she notes.
But her interactions with pharmaceutical companies about new possible targets she has identified have been disappointing, she adds: “They say the bacteria will become resistant.” Indeed, in a clear example of ribosomal variation that is helpful — not to us, but to the pathogen — antibiotics targeting bacterial ribosomes may favor the survival of bacteria with slightly different ribosomes that the antibiotic can no longer block.
This article was originally published on Knowable Magazine.
This article went live on October twenty-ninth, two thousand twenty four, at twenty-four minutes past three in the afternoon.The Wire is now on WhatsApp. Follow our channel for sharp analysis and opinions on the latest developments.




